This guideline has been developed in conjunction with the iHOST team/project - see Intranet for more information/detail
DRUG TREATMENT SERVICES
STARS
Website: https://www.staffstars.org/contact-us/
Contact number: STARS North 01782 639856
Email: [email protected]
With You
Website: www.wearewithyou.org.uk/local-hubs/stoke-on-trent
Contact number: 01782 283113
Email: [email protected]
BACKGROUND
- Patients who use heroin (or other illicit opioids) or receive community prescription for opioid substitution treatment (OST) for opioid dependence are at risk of experiencing withdrawal symptoms when admitted to hospital
- Opioid withdrawal in hospital settings can compromise medical management and lead to discharge against medical advice with associated morbidity and mortality
- Healthcare providers can mitigate physical symptoms and associated risks of opioid withdrawal by ensuring rapid and equitable access to OST [usually oral methadone (full agonist) or sublingual buprenorphine (partial agonist)] for patients with substance dependency:
- if patient(s) in receipt of community prescription for methadone or buprenorphine can confirm routine administered dose, this can be prescribed in hospital to ensure continuity of care
RECOGNITION AND ASSESSMENT
Physical examination
- Signs and symptoms of opioid withdrawal (Table 1); consider assessment using validated scoring tool [e.g. clinical opioid withdrawal scale (COWS/Table 3)]
- COWS score ≥5 indicative of opioid withdrawal – will increase over time without opioid treatment in opioid dependent people
- consider prescribing OST when COWS score ≥5, assessed in conjunction with clinical history and presentation
- document any decision not to prescribe and regularly review e.g. by repeat COWS Score and review
- Patients receiving OST - monitor for signs of opioid toxicity 4 hr after every dose given (Table 2) and then follow NEWS
- Look for and treat signs of injecting drug use e.g.:
- abscess and other skin and soft tissue infections
- venous thromboembolism
- subacute bacterial endocarditis
Table 1: Signs and symptoms of opioid withdrawal
| Early signs and symptoms | Restlessness, anxiety, agitation, discomfort, yawning |
| Intermediate signs and symptoms | Sweating, runny nose, teary eyes, hot/cold flushes, dilated pupils, irritability, loss of appetite |
| Late signs and symptoms | Muscle spasms, insomnia, abdominal cramps, low-grade fever, nausea and vomiting, tachycardia, hypertension, diarrhoea, tremors, goosebumps, bone and muscle aches |
Table 2: Signs of opioid toxicity
| Moderate toxicity | Difficulty keeping eyes open (sedation), drowsiness, shallow breathing, constricted pupils |
| Severe toxicity | Coma, reduced respiratory rate, bradycardia, hypotension, hypothermia |
Guidance for establishing patient opioid history:
- Clarify community OST prescription:
- medication
- formulation
- dose
- frequency of pick-up
- if prescription supervised
- whether patient has brought doses with them, and if so, quantity
- Other opioid use (including in addition to prescribed OST):
- amount
- frequency
- route of administration
- duration of use
- date and time since last dose (if within last 72 hr)
- onset of withdrawal symptoms approximately
- 4 hr after last heroin dose or
- 24 hr after last methadone dose
- Experience of withdrawal symptoms (current and past)
- Concurrent drug/polysubstance use
- amount
- frequency
- route
- duration
- current experience of withdrawal symptoms (if any)
Sources of information
- Following may be used to confirm opioid dependence but not necessarily dose:
- patient self-report (note family/carer self-report can be useful but equally family may be unaware of patient’s drug use or prescribed medication)
- recent previous hospital notes
- recent discharge summaries
- recent drug charts from other hospitals/other clinical settings; but bear in mind since last admission routinely prescribed doses of OST may have changed/patient may have lost tolerance to dose
- summary care records (SCR) or health information exchange (HIE)
- OST doses may frequently change, especially during initial treatment, and drug treatment services do not have write access to SCR to input/update dosing information
INVESTIGATIONS
- Patient history to determine opioid dependence and physical examination to identify presence of withdrawal symptoms is sufficient to indicate OST provision
- If patient has community prescription or history/presentation/supporting evidence consistent with opioid dependency, drug tests e.g. urine drug screens, unlikely to offer additional confirmation/clinical indication of whether OST required
- Drug screens:
- designed to confirm recent opioid use, not prove opioid dependence
- patient consent required
- over-reliance and possible false negatives may compromise timely and appropriate OST
- patients may experience drug screens as stigmatising
- if drug screen requested to enable further targeted discussion/in cases of considerable uncertainty regarding current opioid use, explain why testing required
- make clear appropriate and timely OST dose will be provided and treatment of withdrawal symptoms will not be delayed by waiting for test results
Where withdrawal symptoms clearly present, patient’s decision to withhold test sample/inability to give one should not preclude initiation of OST
TREATMENT
All patients
- Naloxone PRN (see Monitoring treatment)
If contraindications DO NOT prescribe OST: head injury, acute respiratory depression, coma
(see BNF for full list)
- Urinary toxicology screen NOT required before prescribing OST
- If dose cannot be confirmed/patient dependent on opioids (e.g. heroin) but not in receipt of community prescription, prescribe methadone or buprenorphine at starting dose (see flowchart) and titrate to therapeutic dose
- If patient does not consent to OST to manage withdrawal, offer symptomatic relief:
- nausea, vomiting and insomnia: promethazine hydrochloride 25 mg oral 12-hrly
- somatic anxiety: propranolol 40 mg oral 8-hrly (contraindicated if patient has COPD/asthma)
- diarrhoea: loperamide 4 mg single oral dose, and if further loose stools, 2 mg (dose to be taken after each loose stool) (maximum dose 16 mg/day). If infective diarrhoea suspected, do not give loperamide
- stomach cramps: hyoscine butylbromide 10–20 mg oral 6-hrly
- pain: paracetamol 1 g oral 6-hrly or ibuprofen 400 mg oral 8-hrly if required
- In the community, stabilised methadone doses range from 60–120 mg, but doses on both sides of this range are routinely used in practice, guided by response
MONITORING TREATMENT
All patients receiving OST in hospital to be routinely monitored for signs of opioid toxicity 4 hr after every OST dose is given, and then as per NEWS
- All patients requiring OST in hospital (including patients on community OST prescription) prescribe naloxone PRN to treat any severe toxicity from OST or illicit opioid use during hospital admission
- Due to cumulative effect of methadone, carefully monitor patient throughout hospital stay every 4 hr, even where doses have not been increased
- If patient shows signs of toxicity, review daily methadone dose and consider dose reduction
NALOXONE IN CASE OF OVERDOSE
- Initial dose naloxone 100–200 microgram IV bolus, every 60 sec
- if IV access not possible administer IM/until IV access secured
- Initial boluses 100 microgram. If no response following 4 x 100 microgram boluses, subsequent boluses to be 200 microgram
- Continue titrating up to maximum of 2 mg IV until adequate response achieved
- If still no response after total 2 mg, give further 2 mg IV dose
- patients with severe poisoning may require 4 mg dose
- Failure to respond to total dose naloxone 10 mg – alert medical team to assess for alternative causes (e.g. co-administration of alternative CNS depressant drug/brain injury)
- Treatment should aim to reverse respiratory depression, not full reversal of unconsciousness
- Review diagnosis; if respiratory function deteriorates further doses may be required
- Naloxone has shorter half-life than methadone and other opioids
- patient may experience recurring symptoms of severe toxicity after naloxone wears off
- if recurring symptoms consider administration of naloxone infusion
- patient may experience recurring symptoms of severe toxicity after naloxone wears off
- Monitor closely for signs of recurrent overdose for 24 hr
- multiple naloxone administrations may be required
- NOTE: naloxone can precipitate severe opiate withdrawal
- See Toxbase for further advice
Flowchart: OST prescribing
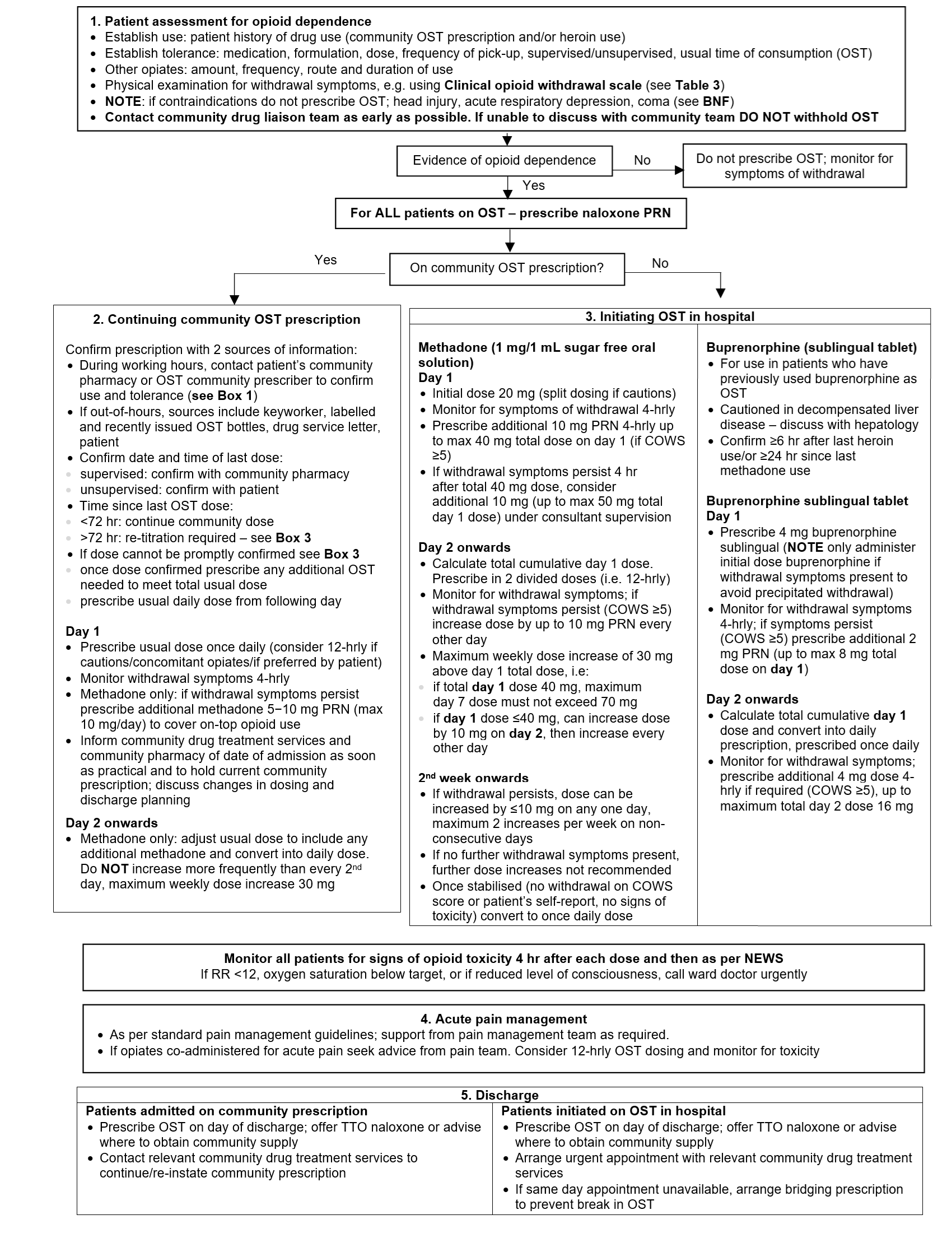
ACUTE PAIN MANAGEMENT
- Maintenance OST doses do not provide analgesic effect for acute pain
- Patients on OST with high opioid tolerance may also have increased pain sensitivity, requiring larger doses of opioid analgesia
- See Faculty of Pain Medicine guidelines on acute pain management for people who receive OST
For patients taking methadone
- Split routine dose and administer 12-hrly or 8-hrly (to allow for monitoring of opioid toxicity symptoms)
- Titrate additional opioid analgesia against acute pain symptoms, monitoring for toxicity symptoms
For patients taking buprenorphine
- Split routine dose and administer 12-hrly or 8-hrly (to allow for monitoring of opioid toxicity symptoms)
- Titrate additional opioid analgesia against acute pain symptoms
- Buprenorphine is a partial opioid antagonist, so may reduce effectiveness of opioid analgesics
- If analgesia insufficient to manage acute pain symptoms, additional non-opioid analgesics can be used
- Alternatively discuss with patient option to discontinue buprenorphine and switch to methadone to optimise pain management
- refer to specialist for further advice and support
DISCHARGE AND FOLLOW-UP
All patients
- On day of discharge, administer patient’s full methadone or buprenorphine dose in hospital
- Offer TTO naloxone where able/refer to local drug and alcohol services
Patients on a community OST prescription
- Advanced discharge planning essential to ensure continuity of care (can represent ‘pinch point’ for transferring patients back into the care of community drug treatment services)
- Prioritise discharge planning as much as possible – include clear communication with patient’s community prescriber and community pharmacy to ensure prescription continuity from day after discharge
- Inform community treatment services of any changes to methadone or buprenorphine dose
- Where possible discharge in-hours to ensure seamless transfer of care from hospital to community drug treatment service
- If in-hours discharge not possible ensure arrangements made to continue prescription in community from day after discharge
Patients initiated on methadone or buprenorphine sublingual in hospital
- Hospital discharge can be a high-risk period for patients who use opioids
- Encourage patients to consider continuing methadone or buprenorphine on discharge, as this can reduce their risks. Not every patient will choose to do so
- Inform community treatment service of day of discharge (in advance if possible), so they can plan for continuity of care from hospital OST to community OST
- Provide copy of discharge summary and confirmation of inpatient prescribing to drug treatment services
BUPRENORPHINE SUBCUTANEOUS (BUVIDAL®)
Long-acting subcutaneous injection
- Only to be initiated by a specialist service
- Licensed for the treatment of opioid dependence within a framework of medical, social and psychological treatment in adults and adolescents aged ≥16 yr
- Patient prescribed Buvidal® will be issued an alert card containing appointment and prescriber details
strongly advised to carry this on their person upon commencing treatment - Available in 2 different formulations which allow administration at either weekly (8 mg,16 mg, 24 mg, 32 mg) or monthly (64 mg, 96 mg, 128 mg, 160 mg) intervals
Clinical management
- Ask to see Buvidal® alert card
- Hospital staff to contact CDAS to obtain clinical handover and allow discussion with CDAS clinician to provide advice and agree ongoing clinical plan for patient
- Contact patient’s community pharmacy to confirm prescribing of Buvidal®
- community pharmacy can advise most recent dose and planned date of administration, however, cannot confirm if Buvidal® was administered
- Buvidal® injection will only be administered by CDAS clinicians
- If CDAS service closed, and patient experiencing significant opioid withdrawal, assess patient using COWS
- conduct full assessment obtaining information relevant to diagnosis and management of patient with drug dependence (see Guidance for establishing patient opioid history)
- where confirmation from community pharmacy obtained and patient assessed with significant withdrawal (COWS >10), low doses of buprenorphine sublingual tablets (4-8 mg, maximum 8 mg daily) can be used ONLY until clinical advice obtained from CDAS
- prescribe naloxone PRN
- DO NOT PRESCRIBE METHADONE
- If no clinical symptoms of opiate withdrawal DO NOT commence on OST regimen
- Buvidal® is long-acting and therefore unlikely patient will experience clinically significant withdrawal due to long half-life
- long-acting nature of Buvidal® can make treating suspected opiate toxicity significantly challenging. Withdrawal can be unpleasant, where opiate toxicity has the potential to be fatal. If uncertainty or discrepancies in information when last dose was administered, withhold OST until discussion with CDAS clinician
SEDATIVE WITHDRAWAL
- Benzodiazepines and other sedative hypnotic drugs
- Alcohol - see Alcohol withdrawal guideline
Symptoms and signs
- Confusion
- Nystagmus
- Tremor
- Agitation, irritability
- Insomnia
- Pyrexia
- Hyperreflexia
- Weakness
- Convulsions
Immediate treatment
- In initial stages, sedative withdrawal symptoms present similarly to alcohol (see Alcohol withdrawal guideline)
- Once symptoms controlled, change to long-acting benzodiazepine (chlordiazepoxide, diazepam) in an equivalent dose (Table 4) to maintain clinical state
- Discuss longer-term strategy with either local drug management service or patient’s GP
Table 4: Equivalent dosages
| Drug | Dosage |
| Chlordiazepoxide | 12.5 mg |
| Diazepam | 5 mg |
| Loprazolam | 500 microgram - 1 mg |
| Lorazepam | 500 microgram |
| Oxazepam | 10 mg |
| Temazepam | 10 mg |
| Nitrazepam | 5 mg |
| Lormetazepam | 500 microgram - 1 mg |
GAMMA-HYDROXYBUTYRATE (GHB)
- GHB is a 'party' drug used for its euphoric effects. It may interact with other illicit/prescribed drugs (e.g. anti-convulsants or anti-psychotics). Long-term use may lead to withdrawal symptoms
Serious side effects
- Headaches
- Hallucinations
- Dizziness
- Confusion
- Nausea
- Vomiting
- Drowsiness
- Agitation
- Diarrhoea
- Sexual arousal
- Numbing of legs
- Vision problems
- Tightness of chest
- Mental changes
- Combativeness
- Memory loss
- Serious breathing and heart problems
- Seizures
- Coma
- Death
Management
- Patients may present to A&E in an intoxicated or comatose state
most wake up within a few hours, but some require ventilation - Due to short half-life, withdrawal symptoms require active management - use diazepam as indicated in Alcohol withdrawal guideline using CIWA-Ar assessment chart, higher doses may be required
- Refer to local community and alcohol service
STIMULANT WITHDRAWAL
- There are no acute symptoms of stimulant withdrawal that need medical treatment as a matter of urgency. Insomnia and anxiety can be treated symptomatically
- Advice and support are valuable
- Depressive symptoms sometimes occur as a later withdrawal effect and can be treated with an antidepressant
- Refer to local community and alcohol service
VOLATILE SUBSTANCES
- Commonly misused are butane, toluene, glues, petrol
- As there are no physical withdrawal syndromes, it is best to discontinue use abruptly
- Treatment of intoxication involves general supportive measures
- Refer to local community and alcohol service
CANNABIS
- Treat anxiety and insomnia symptomatically
Last reviewed: 2026-02-04