BACKGROUND
- Human error is responsible for 1 in 3 transfusion related deaths
- Use Positive Patient Identification (PPID) at every step of the transfusion pathway to uphold patient safety
- ask patient to state his/her name and date of birth whilst checking against patient's wristband
- Encourage patients to report any symptoms experienced during or following a transfusion (including jaundice or dark urine post transfusion)
- Inform all patients who have received blood components or products that they can no longer be a blood donor
- Document any transfusion, including adverse events, on discharge letters
PREVIOUS ALLERGIC TRANSFUSION REACTIONS
- ATRs occur during or <24 hr following a transfusion
- Transfusion associated circulatory overload (TACO) is the commonest cause of morbidity and mortality relating to transfusion
- All patients must have their observations assessed and recorded within 60 min prior to transfusion, at 10-15 min after commencement, and <60 mins of completion
- if patient unable to report potential ATR symptoms, record more frequently
Symptoms and signs of an ATR
- Fever and related inflammatory symptoms or signs e.g.
- chills
- rigors
- myalgia
- nausea or vomiting
- Cutaneous symptoms and signs including urticaria (hives), other skin rashes and pruritus
- Angioedema (localised oedema of the subcutaneous or submucosal tissues), which may be preceded by tingling
- Respiratory symptoms and signs including:
- dyspnoea
- stridor
- wheeze
- hypoxia
- Hypotension
- Pain
- Severe anxiety or 'feeling of impending doom'
- In an unconscious patient, first indication of ATR may include:
- tachycardia
- hypotension
- bleeding
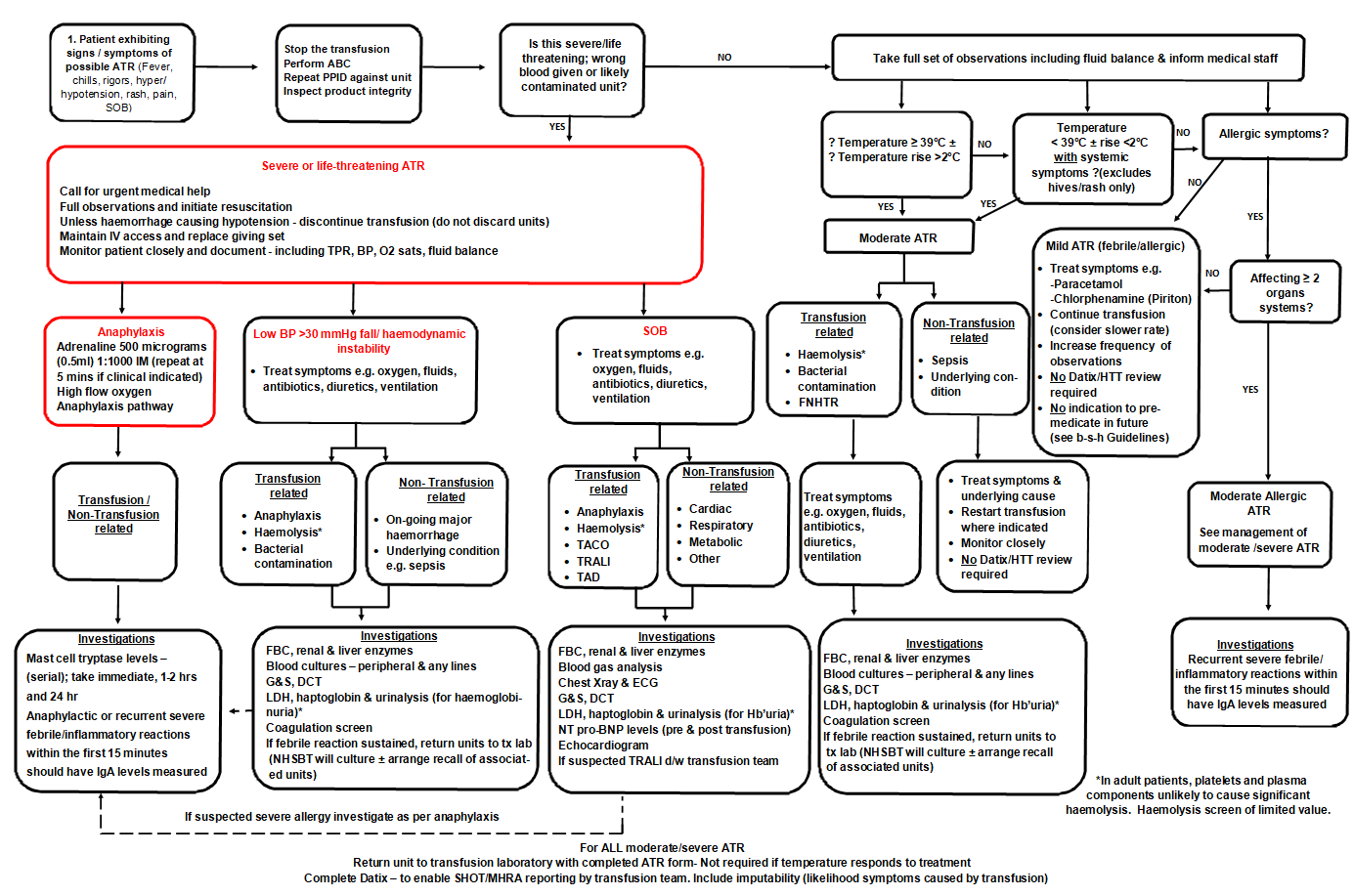
MANAGEMENT OF SUSPECTED ATR
- Perform full set of observations including fluid balance
- Undertake rapid clinical assessment - categorise ATR according to symptom severity
- Check patient ID/blood compatibility label - confirm right patient/right blood
- Visual assessment of blood component - assess risk of bacterial contamination
- Treat symptoms
- Follow Recognition and Primary management of ATRs flowchart below to:
- establish likelihood of ATR vs non-transfusion related symptoms
- establish type of ATR and onward investigations (where clinically indicated)
TYPES OF ATR
Pulmonary complications
- Transfusion associated circulatory overload (TACO) is the commonest cause of morbidity and mortality relating to transfusion
- Very rarely, transfusion related acute lung injury (TRALI) is seen, more commonly following plasma products
Allergic reactions
- Most commonly occur with platelets (particularly apheresis platelets) and plasma
- Occur early during transfusion (within 15 mins for mild allergic, often within few minutes for severe)
- Increased incidence in patients with a history of hay fever (but not causal)
- Mild in >80% - affecting skin only e.g. rash, itching, hives
- Risk of recurrence very low (approximately <1:50 and even in 'frequent reactors' <1:20 - although reactions may cluster)
Anaphylactic reactions affecting ≥2 organ systems
- Can vary in severity from mild to life-threatening (vast majority the former) - categorised as moderate or severe
Bacterial contamination or haemolytic reaction
Consider bacterial contamination or a haemolytic reaction:
- If patient develops sustained febrile symptoms or signs of moderate severity (e.g. temperature ≥39°C or rise of ≥2°C from baseline) and/or
- Systemic symptoms e.g.
- chills
- rigors
- myalgia
- nausea or vomiting
- If reaction transient and patient recovers with only symptomatic intervention, then bacterial contamination/haemolytic reaction very unlikely
Congenital IgA deficiency
- Congenital IgA deficiency (IgA <0.07g/L) +/- IgA antibodies may be related to increased risk of ATR including allergic reactions and rapid-onset inflammatory symptoms (fever, rigors, myalgia <15 mins) but evidence is weak
- if no transfusion history (or history of anaphylaxis), give standard components with increased frequency of monitoring
- otherwise discuss with clinical haematology +/- immunology
Febrile non-haemolytic transfusion reaction (FNHTR)
- Diagnosis of exclusion
REPORTING ATR
- If patient experiences moderate/severe ATR, complete Datix
- All moderate/severe ATR need to be reported to Medicines and Healthcare products Regulatory Agency (MHRA)
- If mild ATR, or if moderate symptoms during transfusion likely attributable to non-transfusion cause, no Datix required
- Document in medical notes any possible ATR (irrespective of severity)
INFORM TRANSFUSION LABORATORY
- If suspected ATR with sustained febrile symptoms or signs of moderate severity (temperature ≥39°C or rise of ≥2°C from baseline and/or systemic symptoms such as chills, rigors, myalgia, nausea or vomiting) to facilitate haemolysis and bacterial screening +/- request product recall by NHS Blood and Transplant (NHSBT)
- If suspected transfusion related acute lung injury (TRALI) - to co-ordinate product recall by NHSBT and appropriate testing
RECOGNITION AND PRIMARY MANAGEMENT OF ATRS
Primary management of ATR including differential diagnosis and investigation
SUBSEQUENT MANAGEMENT OF PATIENTS FOLLOWING ATR
- Risk of recurrence of allergic reactions is very low (approximately <1:50 and even in 'frequent reactors' <1:20 – although reactions may cluster)
- Premedication with antihistamines ineffective as primary or secondary prophylaxis for allergic reactions [randomised controlled trial (RCT) data with diphenhydramine]
- If recurrent moderate/severe allergic reactions, antihistamine prophylaxis can be offered (evidence of efficacy low but risk also low)
- suggest non-sedating antihistamine e.g. cetirizine, especially where patient known to have side-effects from chlorphenamine
- There is no role for hydrocortisone premedication. Steroids thought to be useful to suppress late phase reactions (so no role in anaphylaxis prophylaxis)
- Premedication with paracetamol for patients with repeat FNHTR show conflicting evidence regarding benefit
- paracetamol does not prevent inflammatory symptoms (chills and rigors)
- For all patients use alternatives to transfusion wherever possible and uphold patient blood management strategies
- For management of future transfusions in patients with history of ATR see Table 1: Management of future transfusions in patients with history of ATR
Table 1: Management of future transfusions in patients with history of ATR
| ATR type | Severity/specifications | Management of future transfusions |
| FNHTR | Single |
|
| Recurrent |
Trial of paracetamol 500-1000 mg given 1 hr pre-transfusion
|
|
| Predominately chills and rigors |
|
|
| Allergic reaction | Mild |
|
| Recurrent mild |
|
|
| Recurrent moderate |
|
|
| Severe |
|
|
| Associated with confirmed IgA deficiency3 |
|
1Discuss with clinical haematology who will liaise with NHSBT consultant
2Ideally offer non-sedating antihistamine e.g. cetirizine over chlorphenamine. Consider addition of an H2 receptor antagonists e.g. ranitidine
3Patients with known IgA deficiency (IgA < 0.07g/L) and no history of reactions to blood must be assessed on an individual basis, taking into account the urgency of transfusion, the indication for IgA testing, the anticipated frequency of transfusion, and history of allergy/anaphylaxis in other settings. Most will receive standard components without problems but discussion with a transfusion medicine +/- clinical immunology or allergy specialist is advisable if time allows
DELAYED TRANSFUSION REACTIONS AND LONG-TERM COMPLICATIONS OF TRANSFUSION
Delayed adverse reactions
- Delayed haemolytic transfusion reactions (DHTR) occur >24 hr after transfusion in a patient who has been previously allo-immunised to a red cell antigen by blood transfusion or pregnancy
- Risk of allo-antibody formation is approximately 1:100 although higher if an allo-antibody has already formed
- Viral transfusion-transmitted infections are now very rare in developed countries
- The risk of transmission of variant Creutzfeldt-Jakob disease (vCJD) is negligible and special requirements for patients born after 1.1.1996 were removed by SaBTO in 2019
- However, all patients who have received blood components or products must be informed that they can no longer be a blood donor
Prevention
- Timings of group and screen (G&S) samples should reflect patient's current immune status
- although allo-antibodies may be undetectable in pre-transfusion screening
- Provide irradiated blood components where necessary to prevent transfusion-associated graft vs host disease (TaGVHD)
- where viable lymphocytes engraft and mount a fatal immune response in susceptible patients
- Offer extended Rh/K matched red cells to patients with inherited haemoglobinopathies on chronic transfusion programmes
Symptoms and signs
- DHTR
- occurs <14 days post transfusion
- jaundice
- fever
- anaemia/poor increment in Hb
- haemoglobinuria
- possibly renal failure
- Post transfusion purpura (PTP)
- occurs 5-12 days post transfusion
- bleeding
- thrombocytopenia
- Post transfusion viral infection
- symptoms/signs of infection
- viral transfusion transmitted infections are now very rare in developed countries
- confirmation depends on extensive testing
- Transfusion associated graft vs host disease (TaGVHD)
- occurs typically 7-14 days post transfusion
- fever
- rash
- diarrhoea
- liver dysfunction
- cytopaenia
- fatal
- Iron overload
- occurs over years
- iron deposition in liver, heart and endocrine organs resulting in organ failure
- Allo-antibody formation
- nil - but may have implications for future transfusion practice and pregnancy outcome